Elemental metals react to produce more stable oxide and sulphide forms. In the presence of air, hydrogen sulfide, and moisture, iron reacts to produce various compounds of complex iron sulphide(s), depending on the precise metallurgy of the process system.
More precisely, studies have suggested that a minimum range of 100 ppm of water and an air-to-H2S ratio not exceeding 2.5 to 1 is required for the formation of pyrophoric iron sulfides.
Depending on the thermodynamics of the reaction, the base metal may not always corrode to form sulphides, but may otherwise react to form insoluble oxides (or hydroxides), such as Fe2O3, FeOOH, Fe(OH)2, and Fe(OH)2.
The overall accumulation of pyrophoric deposits in refinery process equipment may be attributed to the corrosion activity of the equipment and will form blackish colored scale(s) or powder(s), that are insoluble in water or oil.
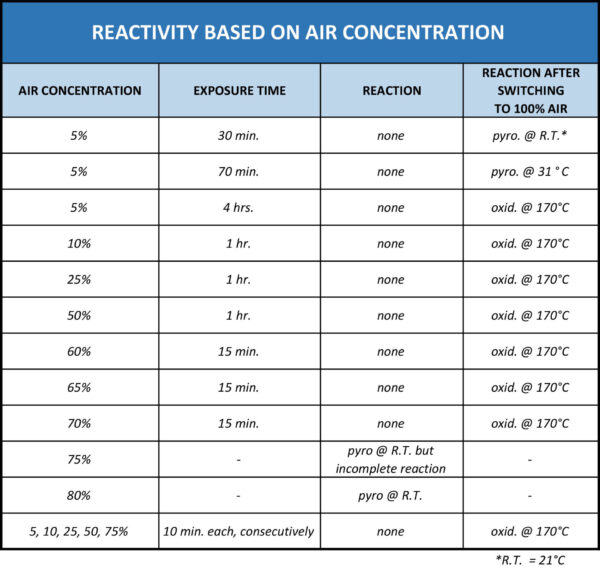
The reactivity of these deposits is dependent on temperature, but concentrations of 2% H2S may generally be considered as sufficient for forming pyrophoric material that may be reactive at ambient temperature. An air concentration of at least 75% is also required before a pyrophoric reaction occurs at ambient temperature. This is equivalent to an oxygen concentration of 15-16%.
The overall reactivity based on air concentration is outlined in the table below.
The use of ROC 40 or ROC 60VP water wets the surfaces of fouled refinery process equipment, removing hydrocarbon deposits, oils, and organics from the iron sulfide deposits and metals surfaces. Any remaining loosened organics or sludge may then be removed by rinsing with hot water while simultaneously eliminating the exothermic reaction between iron sulfide deposits and the atmosphere.
Overall, the specialized formulations of ROC 40 or ROC 60VP serve a dual purpose for refinery cleaning applications. The blend of surfactants improves the wetting ability of either steam or water cleaning applications. This leads to the formation of hydrated iron sulphide species which eliminates the exothermic reaction between pyrophoric iron sulfides and the atmosphere:
FexSx+1 + XH2O → FexSx+1.XH2O
The proprietary oxidizers in ROC 40, for example, also effectively treat hydrogen sulfide and iron sulfide species by an irreversible oxidizing reaction:
2 H2S + O2 → 2 H2O + 2 S
4 FeS + 3 O2 → 2 Fe2O3 + 4 S
Therefore, the suggested chemical cleaning treatments would include a chemical cleaning program using ROC 40 or ROC 60VP to remove oily deposits, then a dilute solution of potassium permanganate or other suitable oxidizer, followed by rinsing and neutralizing.

